A young man was hospitalised with acute abdomen and signs of pancreatitis. He became seriously ill and required surgery to address the underlying cause.
A man in his mid-twenties was admitted to the department of gastroenterological surgery with a 24-hour history of colic-related pain under the right costal arch triggered by eating. He had experienced similar episodes of pain over the past two years, and had been diagnosed in the primary health care service with biliary colic and treated with over-the-counter painkillers. He used no regular medications or dietary supplements, had no allergies, and reported moderate alcohol consumption. On admission, he was afebrile with stable vital signs: a regular pulse of 86 beats per minute, blood pressure of 136/86 mmHg, respiratory rate of 26 breaths per minute, and 100 % oxygen saturation. However, he appeared weak and unwell and required opioid analgesia, and his skin and sclera were visibly jaundiced. Physical examination revealed tense abdominal muscles with tenderness to palpation under the right costal arch and in the epigastrium. Examination of other organs was normal.
Based on the medical history and clinical presentation, biliary colic with secondary incipient cholecystitis or cholangitis was suspected, and the patient was referred for urgent ultrasound examination of the liver, gallbladder and pancreas.
This suspicion was strengthened when preliminary blood tests upon admission revealed a haemoglobin level of 16.1 g/dL (reference range 13.2–16.5) and mean corpuscular volume of 82 fL (83–97). There was significant leukocytosis, with a leukocyte count of 40.3 × 109/L (3.9–9.8 × 109). His C-reactive protein (CRP) level was normal at 1.6 mg/L (0–7), while bilirubin was 201 μmol/L (5–25), alanine aminotransferase (ALT) 84 U/L (10–70), lactate dehydrogenase (LD) 301 U/L (103–213), and gamma-glutamyl transferase (gamma-GT) 226 U/L (10–80). Serum lipase was elevated at 13 281 U/L (4.1–71). The ultrasound examination revealed stones in the gallbladder, dilation of the common bile duct (8 mm, at the level of the pancreatic head, normal range ≤ 6 mm), and encapsulated fluid collections in the upper right quadrant of the abdomen, suggestive of pancreatitis.
Acute pancreatitis can be diagnosed when at least two of the following three criteria are met: acute, persistent epigastric pain, often radiating to the back; elevated serum lipase or amylase (more than three times the upper reference values); and typical findings on diagnostic imaging (CT, MRI, ultrasound) (1). Our patient met all three criteria and was therefore admitted to the department of gastroenterological surgery with suspected acute gallstone pancreatitis. Fluid therapy was initiated, and a pain management plan was established. Magnetic resonance cholangiopancreatography (MRCP) was also performed due to clinical jaundice and elevated gamma-GT and bilirubin – indicating biliary stasis – in the absence of fever or signs of bacterial infection. MRCP was considered indicated to confirm the presence of a stone in the deep bile duct prior to endoscopic removal of a presumed bile duct obstruction.
MRCP was performed the same day and revealed peripancreatic fluid collections and a 4 mm stone in the distal common bile duct (Figure 1). There were also pronounced signs of pancreatitis with surrounding oedema and fluid collections in the upper abdomen. By day 3 after admission, the patient's condition had worsened, and he was experiencing nausea and increased pain. Blood tests showed persistent leukocytosis at 45.6 × 109/L. His CRP level had increased to 261 mg/L, and his bilirubin level had increased further to 290 μmol/L. Broad-spectrum intravenous antibiotics were initiated in the form of piperacillin/tazobactam 4 g/0.5 g three times daily due to suspicion of concurrent infection in the biliary tract. The patient was also referred for endoscopic retrograde cholangiography (ERC), which was performed without complications, with papillotomy followed by removal of two black stones from the distal common bile duct. Cholangiography revealed the gallbladder to be full of stones. Later that day, the patient's bilirubin level was found to have decreased to 139 μmol/L.
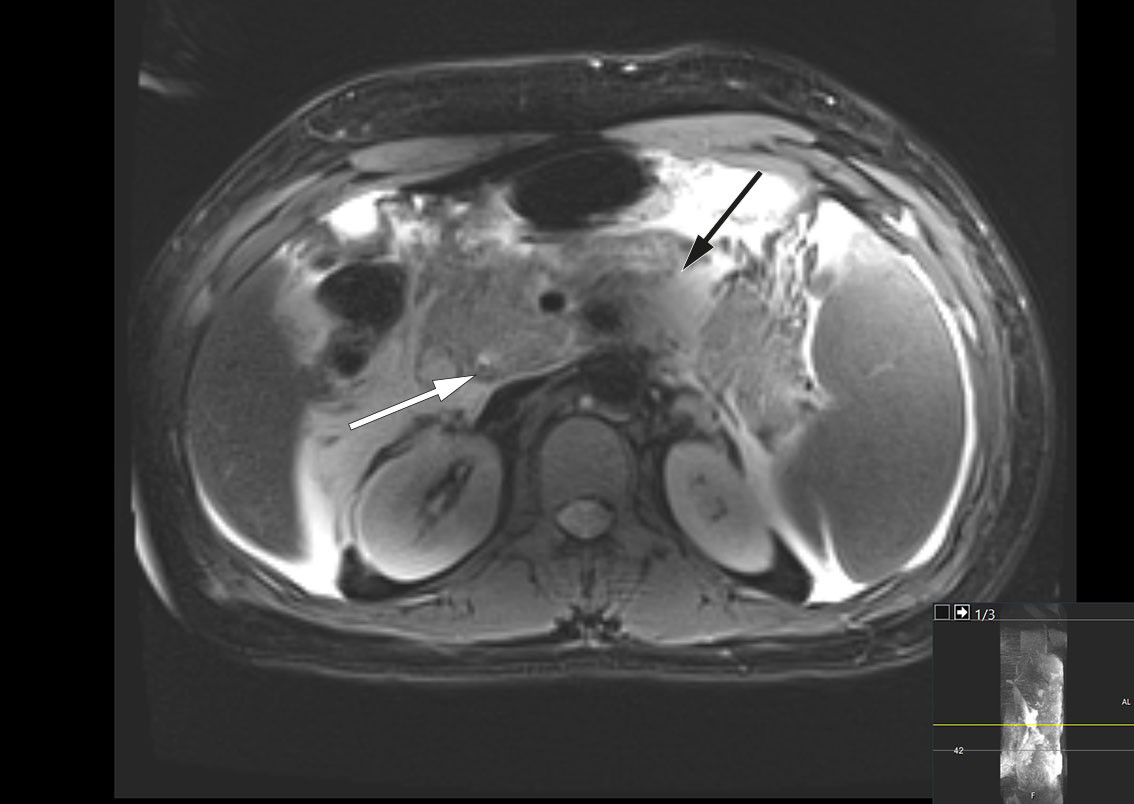
Key differential diagnoses for acute biliary pancreatitis include gallstone disease in the biliary tract, which can lead to infection, particularly acute cholangitis. While antibiotics are not indicated for acute pancreatitis, urgent antibiotic treatment and biliary drainage are necessary in cases of acute cholangitis. Our patient was thought to have acute cholangitis, requiring urgent management, in addition to acute biliary pancreatitis.
Later that day, after the ERC examination, the patient became febrile with a temperature of 38.4°C and increasing tachycardia with a pulse of 110–130 beats/min. His oxygen requirements increased, with 1–2 L of O2 required via nasal catheter to maintain SpO2 > 95 %. Vasopressor treatment was not necessary, but a urinary catheter was inserted due to intensive intravenous delivery of 5,000 mL of Ringer's acetate, resulting in diuresis of 40–50 mL/h. Due to his clinical deterioration, the patient continued to be monitored on the post-surgical ward over the next 24 hours.
Acute pancreatitis has a mild, self-limiting course in almost 80 % of cases, and the main focus of treatment should be symptom relief and fluid therapy (2). Accordingly, however, about one in five patients with acute pancreatitis will have a more fulminant and severe course, in which closer monitoring of vital functions is necessary. This was initiated for our patient on day 3 on the post-surgical ward, as the hospital did not have a separate surgical intermediate care unit.
Over the course of day 3, the patient's clinical condition deteriorated further and he developed severe abdominal pain. He was transferred to the intensive care unit due to incipient organ failure with a need for oxygen. He was somnolent and, although breathing independently, required 2–3 L of O2 via nasal catheter to maintain oxygen saturation above 92 %. His INR level had increased to 1.9 while his albumin level had fallen to 22 g/L (from 45.6 g/L), raising suspicion of a bleeding disorder. In addition, his bilirubin level had increased once again to 290 μmol/L, but in the absence of any other liver or bile enzyme increases, it was decided based on an interdisciplinary discussion not to proceed immediately with another ERC examination. Further diagnostic imaging with a CT scan of the abdomen and pelvis revealed necrotising pancreatitis with peripancreatic fluid collections and free fluid in the abdomen (Figure 2). There were no signs of intra- or extrahepatic cholestasis. However, bilateral pleural effusion and atelectasis were observed, which could explain the deterioration in respiratory function. Due to the patient's elevated INR level, a pleural tap was not performed. Enlargement of the spleen (21 cm in length) was also noted.
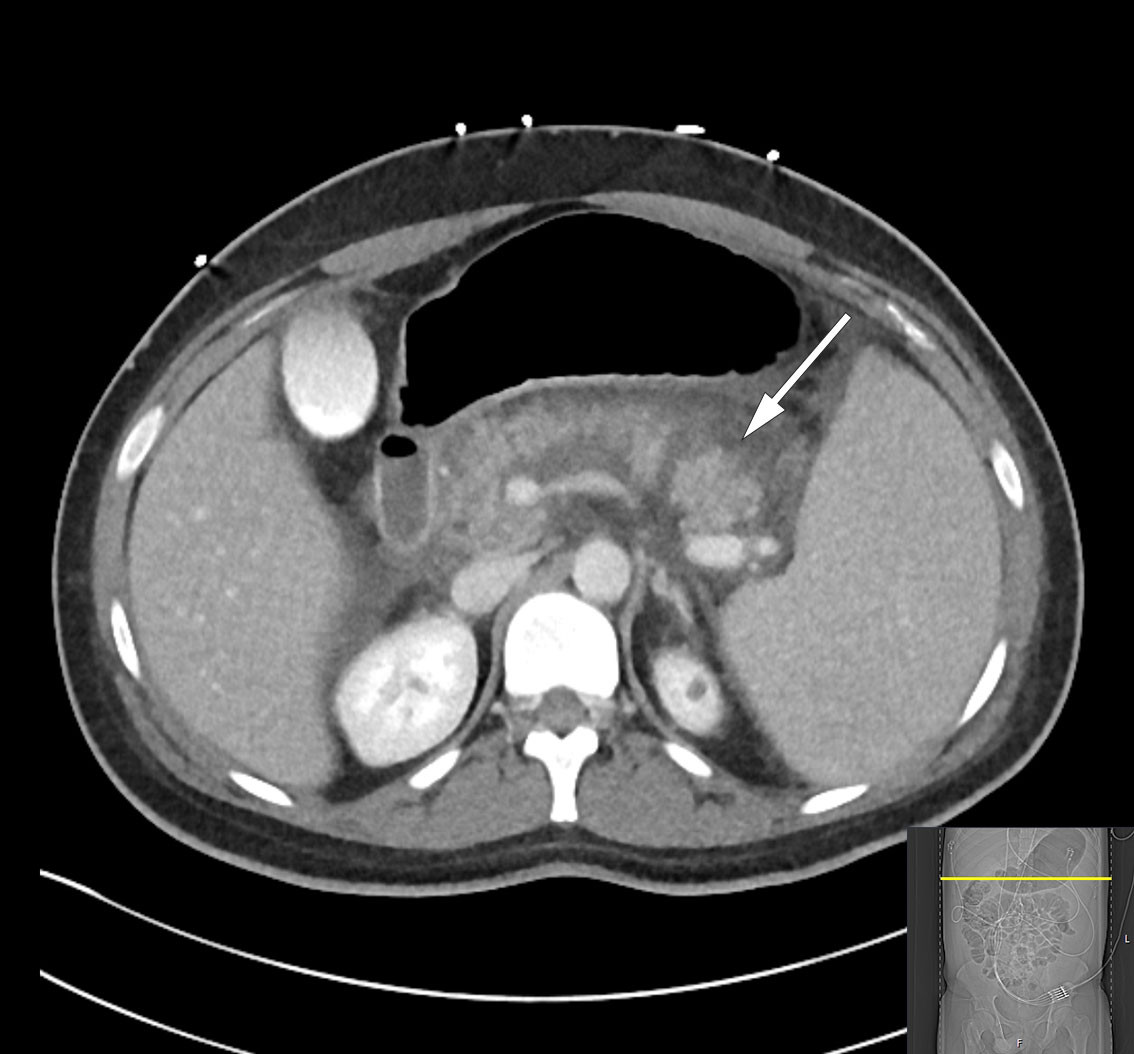
Necrotising pancreatitis is a serious complication of acute pancreatitis and is usually associated with a prolonged and severe clinical course. Necrosis in the pancreatic parenchyma or peripancreatic tissue occurs in 5–10 % of patients with pancreatitis and rarely remains confined to the pancreas (1). Secondary infection of necroses due to bacterial translocation from the gastrointestinal tract is not uncommon, but rarely occurs within the first week. Infection should be suspected when extraluminal gas is visible within the pancreas or in surrounding tissues on CT; broad-spectrum antibiotic treatment is then indicated. Necrosectomy to remove dead tissue is only considered appropriate from 3–4 weeks into the disease course, using a step-up approach with increasing invasiveness (3).
Due to restrictions during the COVID-19 pandemic, family visits had been limited. However, in connection with the patient's transfer to the intensive care unit, telephone contact was established with the attending consultant in gastrointestinal surgery. During this conversation, it emerged that the patient's relatives had reported a family history of spherocytosis.
Splenomegaly is a non-specific sign with various underlying causes (4). Hereditary spherocytosis is the most common form of inherited haemolytic anaemia in Northern Europe and, in 75 % of cases, has an autosomal dominant inheritance pattern (5). The different genetic variants have highly variable clinical penetrance, ranging from transfusion-dependent neonatal jaundice to lifelong asymptomatic status. It is not uncommon for spherocytosis symptoms to first manifest during intercurrent or acute illness. Our patient's blood tests showed mixed hyperbilirubinemia (total bilirubin 478 μmol/L [5–25] and conjugated bilirubin 350 μmol/L [<4]), caused by cholestasis and possible haemolysis. The latter was inconclusive due to normalisation of slightly elevated lactate dehydrogenase (371 U/L, with interference noted due to high bilirubin) and a normal haptoglobin level. However, the occurrence of gallstones at a young age, as well as the patient's family history, were consistent with chronic haemolysis secondary to spherocytosis. A direct antiglobulin test was performed to rule out autoimmune haemolysis, and spherocytosis was confirmed by immunophenotyping using flow cytometry. Considering the diagnosis, future splenectomy was recommended after a haematological consultation.
The patient was monitored in the intensive care unit for four days under epidural analgesia. Another ERC examination was performed, and a stent was placed in the common bile duct to ensure drainage of any migrating gallstones. Nevertheless, a transient increase in bilirubin to 399 µmol/L was observed due to haemolysis, with haemoglobin levels decreasing from 12.6 g/dL to 9.4 g/dL during the stay in the intensive care unit.
The patient gradually improved and was discharged after 18 days. Three days later, however, he was readmitted due to increasing pain and dyspnoea. A CT scan revealed encapsulation of peripancreatic necrotic fluid collections with partial compression of the portal and splenic veins. There was no evidence of cholestasis, and a slight reduction in free fluid was seen in the abdominal cavity. Blood tests showed signs of inflammation, and oral antibiotic treatment was initiated in the form of trimethoprim/sulfamethoxazole 160 mg/800 mg, twice daily, along with metronidazole 400 mg three times daily, due to suspected secondary infection of necrosis. The patient was discharged home without further antibiotic treatment eight days after the second admission. Endoscopic transgastric drainage of encapsulated necrotic fluid collections, known as walled-off necrosis (WON), was planned for the following week, due to compression of the portal and splenic veins.
Three days after being discharged for the second time, the patient was readmitted again with increasing pain in the left abdomen radiating to the back. Blood tests showed leukocytosis with a leukocyte count of 28.1 × 109/L and a CRP level of 7.6 mg/L. His haemoglobin level had decreased slightly to 12.4 g/dL whereas bilirubin had increased to 53 µmol/L. Lipase was very slightly elevated at 81 U/L. On the third night after readmission, the patient was normotensive but tachycardic, with a heart rate of 134 beats/min, respiratory rate of 44 breaths/min, and oxygen saturation of 91 % on room air. His skin was pale and clammy, his breathing shallow, and he was visibly in pain. A CT scan showed increasing ascites but with decreasing size of the largest fluid collection. There was no dilation of the bile ducts, but basal lung consolidations had developed on the left side, along with small amounts of pleural fluid. The latter is not uncommon in light of the proximity of the pancreas to the diaphragm, such that widespread inflammation can affect the pleural cavity. Inflammatory markers were elevated, with a leukocyte count of 22.5 × 109/L and CRP level of 51 mg/L, as well as decreasing haemoglobin of 10.6 g/dL. It was decided to transfer the patient to the intensive care unit for insertion of an epidural catheter to optimise analgesia, and to refer him for urgent drainage of the encapsulated necrosis.
The following day, the patient was febrile with a temperature of 38.5°C but was stable on room air. The encapsulated necrosis was treated by inserting a Hot AXIOS lumen-apposing metal stent (LAMS), a system developed for endoscopic ultrasound-guided drainage of encapsulated necrosis via the stomach. A feeding tube was placed in the small intestine, and nutritional supplementation was initiated. Over the following days, the patient's clinical and biochemical status fluctuated. Broad-spectrum intravenous antibiotics were initiated in the form of meropenem 1 g three times daily due to an increase in CRP to 148 mg/L. In addition, a fall in haemoglobin to 6.9 g/dL led to the decision to transfuse the patient with three units of packed red cells. A CT scan, however, showed a significant reduction in the size of the encapsulated necrosis after placement of the stent, and showed the blood vessels to no longer be compressed (Figure 3). The patient underwent necrosectomy via gastroscopy, with removal of large amounts of dead tissue. Over the next few days, the patient was gradually mobilised, with accompanying improvements in respiration and good diuresis supported by loop diuretics. Two sets of blood cultures were sterile, while Staphylococcus epidermidis was detected in the urine but was of uncertain clinical significance. The epidural catheter was removed, and opioids were tapered in accordance with the patient's increasing mobility. The patient was then transferred to the gastroenterological surgery ward on day 10 of his third admission.
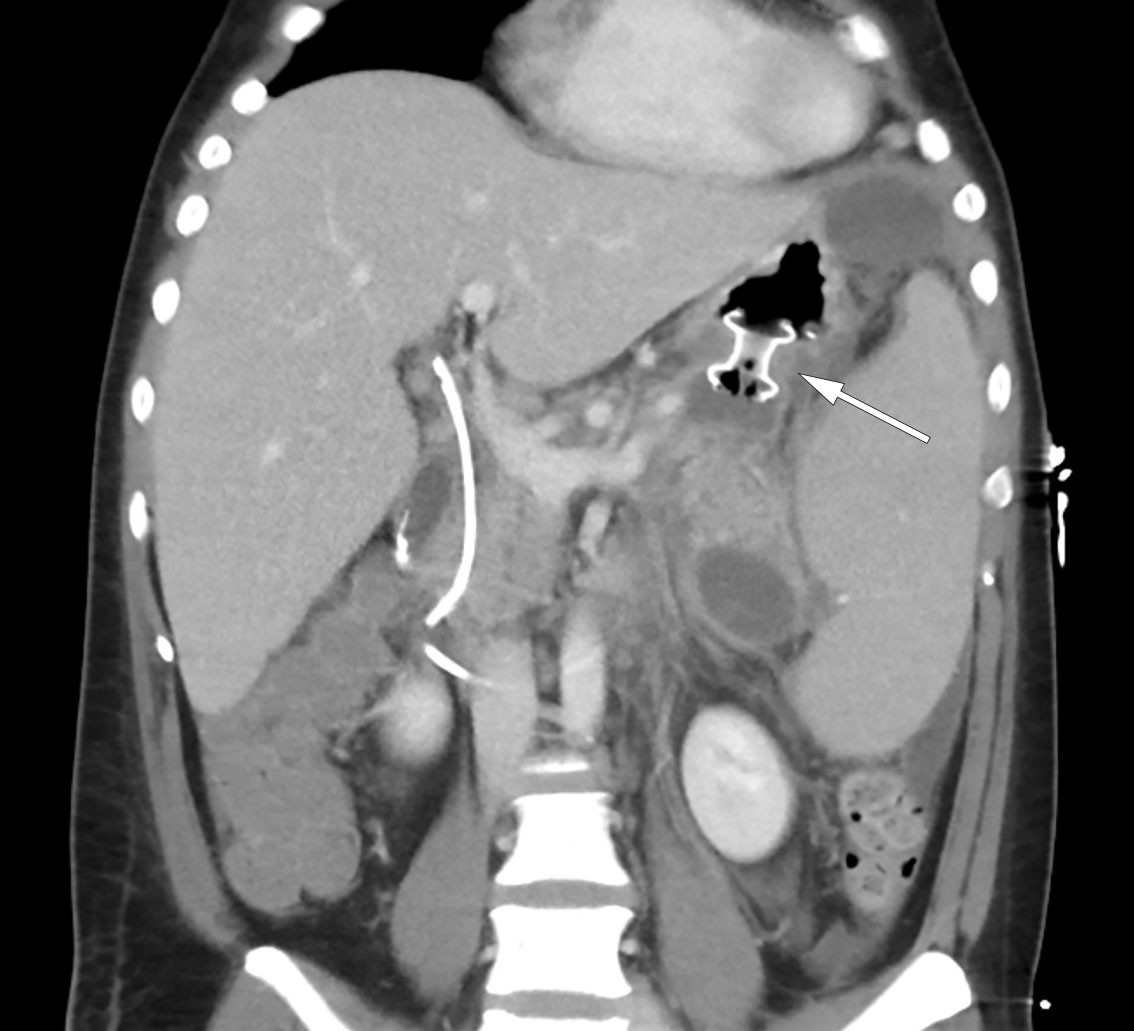
The patient showed gradual improvement. On day 25, the Hot AXIOS stent was removed without complications, and on day 29 of his third admission the patient was discharged. He was pain-free, and his abdomen soft and non-tender to palpation. He was fully mobilised and had resumed a normal diet. Inflammatory markers were normal, although he had mild anaemia with haemoglobin of 9.2 g/dL. He was prescribed proton pump inhibitors, pancreatic enzymes, and folic acid (levels of the latter are often low in chronic haemolysis). The plan for future cholecystectomy and splenectomy remained unchanged. Two weeks after discharge, a CT scan of the pancreas showed no residual effects of the pancreatitis but persistent splenomegaly. Endoscopic removal of the bile duct stent was performed without complications two months after discharge.
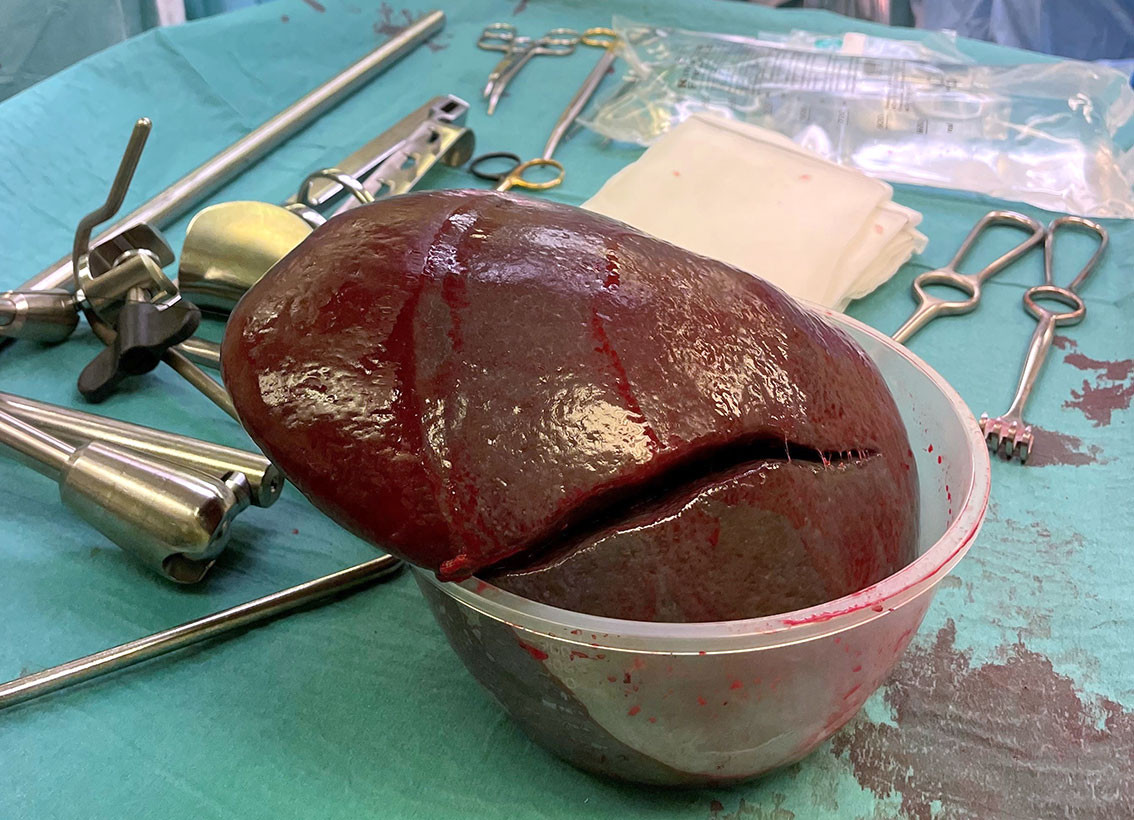
Four months later, the patient was readmitted for surgery. He had an elevated bilirubin level of 81 µmol/L, but other blood tests were within reference ranges. Laparoscopic cholecystectomy and splenectomy were initiated but proved technically challenging owing to adhesions and a markedly enlarged spleen. The procedure was therefore converted to open surgery after the spleen had been detached almost completely via laparoscopy. Total blood loss was in the order of 1,000 mL, with much of this coming from the spleen. The spleen specimen weighed 1,609 g in total (normal weight is 120–200 g) (Figure 4). Subsequent histological analysis showed an incidental finding of benign lymphangioma but no atypia. The postoperative course was uneventful, and the patient was discharged on the eighth postoperative day. Further vaccinations were planned in line with current guidelines for post-splenectomy prophylaxis, and the patient was referred to the haematology department for follow-up with respect to his underlying condition (6).
Eight months after surgery, the patient is doing well. As the risk of haemolytic anaemia following splenectomy is low, even during intercurrent illness, no further follow-up is planned. However, the patient has been offered genetic counselling due to suspected hereditary spherocytosis.
Discussion
Acute pancreatitis is a serious inflammatory condition of the pancreas (7). It can be life-threatening, but most cases have a transient and mild course. Incidence varies geographically, with about 30.6 cases per 100,000 inhabitants in Norway (8). Severe disease occurs in 20 % of patients, with 20–30 % of these cases resulting in death (9). The overall mortality rate is 3 %. The aetiology is diverse, but the most common precipitating factors are gallstones, followed by alcohol use, which together account for 70–80 % of cases. Treatment consists mainly of intravenous fluid administration, pain relief, and nutritional support. In patients with necrotising pancreatitis, treatment may also involve necrosectomy to remove dead tissue via endoscopy. This procedure is typically performed after four weeks to allow the necroses to 'mature' and become encapsulated.
In addition to gallstones and alcohol use, a number of rare conditions can cause pancreatitis. In our patient, pancreatitis was caused by gallstones secondary to haemolytic anaemia that was in turn caused by hereditary spherocytosis (5) (Figure 5). Spherocytosis is a condition in which a defect in the membrane of red blood cells leads to them acquiring a spherical shape. This reduces the elasticity of the cells, which are known as spherocytes, and hinders their passage through the capillaries. In addition, splenic macrophages recognise spherocytes as defective and more frequently remove them from the circulation. Over time, hypersplenism develops, leading to increased destruction of red blood cells and subsequent chronic anaemia. The severity of the disease is therefore related to the proportion of spherocytes in the blood. In addition, the breakdown of haem in haemoglobin results in increased levels of bilirubin, leading to the formation of biliary gallstones in the gallbladder. These then migrate into the biliary tree and can cause biliary colic, which is often self-limiting as the stones pass into the duodenum.
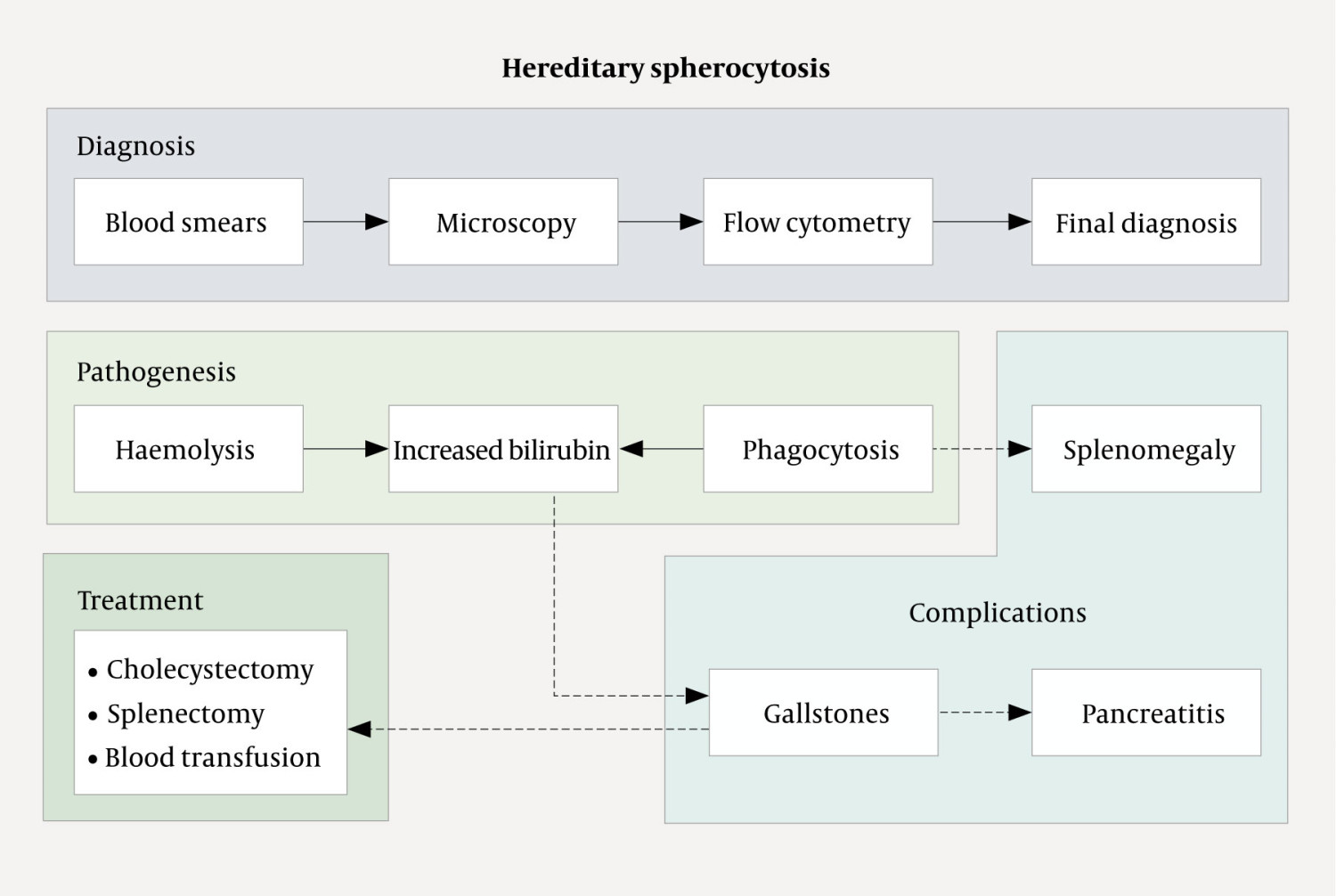
The mechanism by which the passage of gallstones leads to the development of pancreatitis is unknown, but is believed to reflect transient or persistent biliary stasis caused by migrating or impacted gallstones in the bile duct; alternatively, it may be related to the duodenal papilla. The significant reduction in risk of recurrence following cholecystectomy and removal of stones from the biliary system provides support for both causes (10, 11). Our patient ultimately required surgery. If a patient with spherocytosis is only mildly affected by anaemia and gallstones, then splenectomy alone will be sufficient. However, in patients with a history of biliary pancreatitis, for example, there is a clear indication for cholecystectomy to prevent recurrence. Ideally, this risk should be recognised prior to symptom onset, with information about family history being crucial. In our patient, regular follow-up from childhood, with subsequent prophylactic cholecystectomy and splenectomy, would likely have prevented a severe disease course.
Acute pancreatitis is a commonly encountered condition in gastroenterological surgery departments, with most cases attributable to gallstones or to alcohol use. Hereditary spherocytosis is the most common form of hereditary haemolytic anaemia but is rarely a cause of gallstone-induced pancreatitis. Severe jaundice that showed no improvement despite initial biliary drainage and removal of gallstones suggested an alternative underlying cause of elevated bilirubin levels. Spherocytosis should be suspected in cases of comorbid anaemia, a family history of spherocytosis, and the absence of other triggers of pancreatitis.
The patient has consented to the publication of this article.
This article has been peer-reviewed.
- 1.
Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62: 102–11. [PubMed][CrossRef]
- 2.
Mofidi R, Duff MD, Wigmore SJ et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006; 93: 738–44. [PubMed][CrossRef]
- 3.
Trikudanathan G, Wolbrink DRJ, van Santvoort HC et al. Current Concepts in Severe Acute and Necrotizing Pancreatitis: An Evidence-Based Approach. Gastroenterology 2019; 156: 1994–2007.e3. [PubMed][CrossRef]
- 4.
Pozo AL, Godfrey EM, Bowles KM. Splenomegaly: investigation, diagnosis and management. Blood Rev 2009; 23: 105–11. [PubMed][CrossRef]
- 5.
Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet 2008; 372: 1411–26. [PubMed][CrossRef]
- 6.
Kuchar E, Miśkiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171: 683–94. [PubMed][CrossRef]
- 7.
Boxhoorn L, Voermans RP, Bouwense SA et al. Acute pancreatitis. Lancet 2020; 396: 726–34. [PubMed][CrossRef]
- 8.
Gislason H, Horn A, Hoem D et al. Acute pancreatitis in Bergen, Norway. A study on incidence, etiology and severity. Scand J Surg 2004; 93: 29–33. [PubMed][CrossRef]
- 9.
Petrov MS, Shanbhag S, Chakraborty M et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139: 813–20. [PubMed][CrossRef]
- 10.
van Baal MC, Besselink MG, Bakker OJ et al. Timing of cholecystectomy after mild biliary pancreatitis: a systematic review. Ann Surg 2012; 255: 860–6. [PubMed][CrossRef]
- 11.
McAlister VC, Davenport E, Renouf E. Cholecystectomy deferral in patients with endoscopic sphincterotomy. Cochrane Database Syst Rev 2007; 2007: CD006233. [PubMed][CrossRef]