A previously healthy man in his late thirties was admitted to hospital after several days of illness with clinical and radiological findings consistent with COVID-19. Nasopharyngeal swabs taken on days 5 and 7 after symptom onset tested negative, but a bronchoalveolar lavage specimen on day 8 tested positive. This underlines the fact that patients with COVID-19 may have negative nasopharyngeal tests early in the disease course.
A previously healthy foreign-born sailor in his late thirties was hospitalised with chest pain, dry cough, wheezing and acute abdominal pain. He used no medications and had never smoked. He communicated via an interpreter. The patient worked on a ship that had been in port in a non-Nordic country a week earlier. While there, he had not left the ship, but there had been several visitors on board who were coughing. The ship docked in Norway, but the crew were not allowed to disembark. Over the previous 3–5 days he had had fever, chest pain and a reduced sense of taste and smell. On the day of admission, he had abdominal pain, vomiting and reduced general condition. He stated that several other members of the crew were also coughing. The patient was admitted with droplet isolation precautions because of his respiratory symptoms and the ongoing COVID-19 pandemic.
Upon arrival in Acute Admissions, he was awake, alert and oriented. His blood pressure was 139/90 mm Hg, heart rate 110 beats/min and oxygen saturation (SpO2) 88 % in room air. He had circumoral cyanosis and a rapid respiratory rate of 36 breaths per minute, but no apparent dyspnoea. Blood gas analysis showed type I respiratory failure (hypoxemia without hypercapnia) with pO2 of 6.9 kPa (reference range 11.0–14.0) and mild respiratory alkalosis with pH 7.47 (7.37–7.45) and pCO2 4.5 kPa (4.7–6.0). Tympanic temperature was 38.6 °C, but he otherwise appeared well. Clinical examination revealed pronounced tenderness to palpation over the entire thorax, but otherwise normal findings. An ECG showed no signs of ischaemia, and two troponin assays were negative. The chest pain was considered highly unlikely to be of cardiac origin.
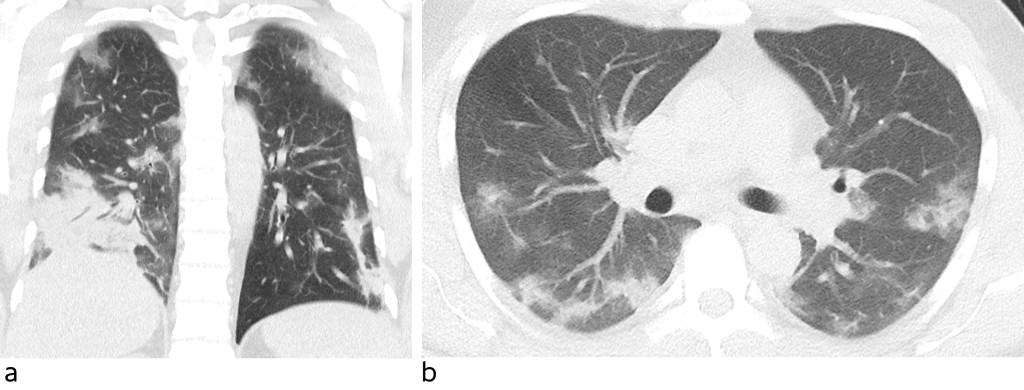
Blood tests showed haemoglobin (Hb) 12.3 g/dl (13–17), normal leukocytes with normal differential count, sedimentation rate (SR) 36 mm (<13), C-reactive protein (CRP) 121 mg/l (<5), albumin 33 g/l (39–50), lactate dehydrogenase (LD) 349 U/l (105–205), creatine kinase (CK) 537 U/l (50–400), aspartate aminotransferase (AST) 47 U/l (15–45), and ferritin 1 192 µg/l (15–350). CT thorax showed abundant diffuse ground-glass opacities in all lobes, interspersed with normal lung parenchyma (Figure 1). Apical changes were most apparent in the periphery. There was also a consolidated opacity basodorsally in the right lower lobe. Five and seven days after symptom onset, nasopharyngeal swab specimens were collected to test for SARS-CoV-2 using polymerase chain reaction (PCR). Both swabs tested negative.
For the first few days the patient was kept in isolation in the intensive care unit with droplet isolation precautions owing to a strong suspicion of COVID-19, silent hypoxemia and a risk of rapid deterioration (1). He received oxygen therapy and had oxygen saturation of 95 % with 4 l oxygen via nasal cannula. He did not require any additional oxygen beyond this, and did not require non-invasive ventilation (NIV). Antibiotic therapy in the form of piperacillin/tazobactam was started to cover the possibility of bacterial agents. The choice of antibiotic was based on the patient's nationality and travel history.
After two negative nasopharyngeal swabs, he was taken out of isolation on day 7. On day 8, he was re-isolated and bronchoscopy with bronchoalveolar lavage (BAL) was performed under light sedation to obtain a specimen from the lower respiratory tract. During the procedure, copious amounts of grey turbid mucous were observed, the patient coughed vigorously and his oxygen saturation rapidly decreased. The specimen tested positive for SARS-CoV-2 and showed a low viral load with exponential amplification that began after 36 cycles (Ct value). The patient was dependent on supplemental oxygen for eight days. He was otherwise in good shape with no obvious dyspnoea, but with circumoral cyanosis and a significant fall in oxygen saturation upon mobilisation. His lowest measured SpO2 was 70 %. He had no other organ failure, and the disease course was otherwise uncomplicated.
Discussion
Our patient, who was young and previously healthy, developed hypoxic respiratory failure requiring more than a week of oxygen therapy. Two sets of nasopharyngeal swabs were negative for SARS-CoV-2, but a specimen obtained with bronchoalveolar lavage tested positive nonetheless.
A similar case report in the Journal of the Norwegian Medical Association reported negative nasopharyngeal swabs after more than three weeks of illness, with the negative results assumed to be due to a low viral load in the upper respiratory tract (2). Our case history shows that nasopharyngeal swabs may yield false-negative results, even at early stages of the disease.
Other international case reports and studies have shown that in the first week after symptom onset, false-negative results from the nasopharynx are common, occurring in close to 30 % of cases (3, 4). It transpires that the viral load in the upper respiratory tract is highest in the week after symptom onset, which was also when specimens were collected from our patient (5). The specimens were collected as recommended, from the posterior pharyngeal wall and the nasopharynx. In accordance with local procedures they were taken to a regional hospital for analysis the following day. The use of bronchoalveolar lavage to obtain specimens is recommended when COVID-19 is suspected but nasopharyngeal swabs test negative (6). The specimen collected via bronchoalveolar lavage did indeed test positive when analysed using the same technique as the two previous specimens from the nasopharynx. Unfortunately, nasopharyngeal swab specimens were not collected on the same day as the bronchoalveolar lavage.
Bronchoscopy with bronchoalveolar lavage is an aerosol-generating procedure. As this is thought to increase the risk of infection for healthcare personnel, use of this procedure requires more extensive personal protective equipment (7). The indication for use must be carefully considered with respect to the potential benefits. For our patient, an accurate test result was important because he was part of a ship's crew and wished to return to his home country. In other situations, the risk of infecting healthcare personnel and the discomfort the procedure entails for the patient may outweigh the benefits. One might then choose instead to isolate the patient on the basis of other findings that strongly suggest COVID-19. Our patient had a travel history that led us to suspect that he may have been exposed to the virus, as well as symptoms (7) and findings on CT thorax that were consistent with COVID-19. CT findings have proven to have high sensitivity for detection of COVID-19, up to 95 %. However, the specificity is low, as other viruses and atypical infections may produce a similar clinical picture (4, 8, 9). Aside from high ferritin levels, blood tests revealed only minor, non-specific abnormalities. Persistently high ferritin levels have been shown in retrospective studies to be a sign of poor prognosis for COVID-19 patients (10).
The patient was removed from isolation after two negative nasopharyngeal swab tests. However, on account of his symptoms and clinical results it was decided to isolate him again the following day, and therefore only a small number of healthcare personnel had to be quarantined. During the COVID-19 pandemic, healthcare personnel are already in a vulnerable position, with respect to both infection risk and staffing levels. We have therefore chosen to involve the specialists responsible for care in decisions on whether to discontinue isolation precautions.
Our case study highlights the importance of combining recent medical history, clinical findings and CT findings before discontinuing isolation of patients with negative nasopharyngeal tests for COVID-19. Specimens collected by bronchoalveolar lavage are more reliable than nasopharyngeal swabs (11) and should therefore be considered in cases of doubt.
The patient has consented to the publication of the article.
We are grateful to the Department of Radiology, Kirkenes Division, Finnmark Hospital Trust for providing the CT images.
- 1.
Ottestad W, Seim M, Mæhlen JO. Covid-19 med stille hypoksemi. Tidsskr Nor Legeforen 2020; 140. doi: 10.4045/tidsskr.20.0299. [CrossRef]
- 2.
Hauge MT, Nilsen E, Nordseth T. Akutt lungesviktsyndrom hos covid-19-pasient med negative nasofarynksprøver. Tidsskr Nor Legeforen 2020; 140. doi: 10.4045/tidsskr.20.0297. [CrossRef]
- 3.
Winichakoon P, Chaiwarith R, Liwsrisakun C et al. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol 2020; 58: e00297–20. [PubMed][CrossRef]
- 4.
Fang Y, Zhang H, Xie J et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020; 295: 200432. [PubMed][CrossRef]
- 5.
Pan Y, Zhang D, Yang P et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020; 20: 411–2. [PubMed][CrossRef]
- 6.
Clinical care for severe acute respiratory infection: toolkit. COVID-19 adaptation. Geneva: World Health Organization, 2020. https://apps.who.int/iris/handle/10665/331736 Lest 7.5.2020.
- 7.
Thomas-Rüddel D, Winning J, Dickmann P et al. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist 2020 doi: 10.1007/s00101-020-00760-3. [CrossRef]
- 8.
Caruso D, Zerunian M, Polici M et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology 2020; 295: 201237. [PubMed][CrossRef]
- 9.
Li Y, Xia L. Coronavirus disease 2019 (COVID-19): Role of chest CT in diagnosis and management. AJR Am J Roentgenol 2020; 214: 1–7. [PubMed][CrossRef]
- 10.
Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [PubMed][CrossRef]
- 11.
Wang W, Xu Y, Gao R et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323. doi: 10.1001/jama.2020.3786. [PubMed][CrossRef]
Marit Teigen Hauge og medarbeidere presenterer et kasus i Tidsskriftets nr. 7 der en pasient med negative nasofarynksprøver utvikler ARS med sars-CoV-19 infeksjon (1). Falsk negative testresultater av PCR-testing har det vært lite fokus på hos helsemyndigheter og presse. En artikkel i Radiology fra tidligere i år viser at det er en erkjent svakhet med testen (2). Det er litt vanskelig å finne ut nevnerne i tallene fra artikkelen, men jeg regner meg fram til at PCR har sensitivitet på 0,71 og CT 0,97. Slik jeg leser artikkelen er det 850 covid-19-syke basert på typisk klinikk og typiske CT-funn. 164 har sannsynlig annen sykdom. Det tar gjennomsnittlig 5 dager fra en negativ PCR blir positiv hos covid-19-syke, ifølge forfatterne.
Det er mye usikkerhet knyttet til pandemien. Validitetsbegrensninger av test-regimet er bare ett av dem. Imidlertid burde den lave sensitiviteten av PCR-testen ført til større skepsis med tanke på å bare teste høyprevalenspopulasjonen, de med kliniske funn som indikerer sykdom. Det gjør det også vanskelig å forstå at myndighetene bare vil gi karantenefritak for dem som har hatt positiv PCR-test, og ikke dem som har positiv immunglobulin-test.
Referanser
1. Hauge MT, Nilsen E, Nordseth T. Acute respiratory distress syndrome in a patient with COVID-19 and negative nasopharyngeal swabs. Tidsskrift for den Norske laegeforening :. 2020;140(7):688-90.
2. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020:200642.